Medical Product Development
We make your medical device fit for use
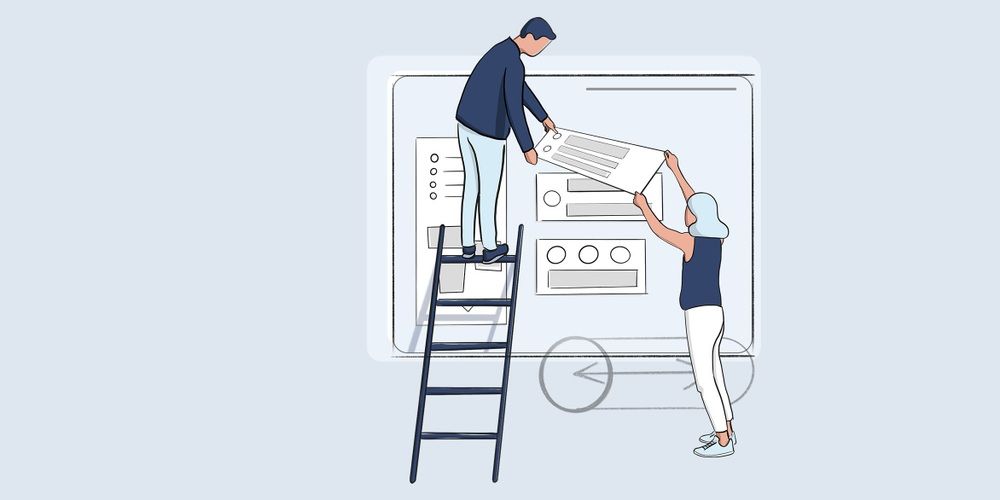
We support you in the implementation of usability for medical devices in accordance with DIN EN 62366-1 as required by the standard. We create use specifications, user interface operating concepts, carry out the use-related risk analysis, plan and carry out formative and summative tests, evaluate these and design your medical device.

Thanks to our many years of expertise in many other sectors, we are also able to incorporate the user experience increasingly demanded by users into medical devices. Products developed by us are not only fit for purpose, they also offer a high degree of positive user experience through modern, customized but characteristic design and gamification.
We also support your project plans in hardware-side product development.
Our range of services
- Product ergonomics & usability
- Product & UX design style guides
- Usability engineering
- Usability testing: formative/summative
- User Experience
- User Interface Design
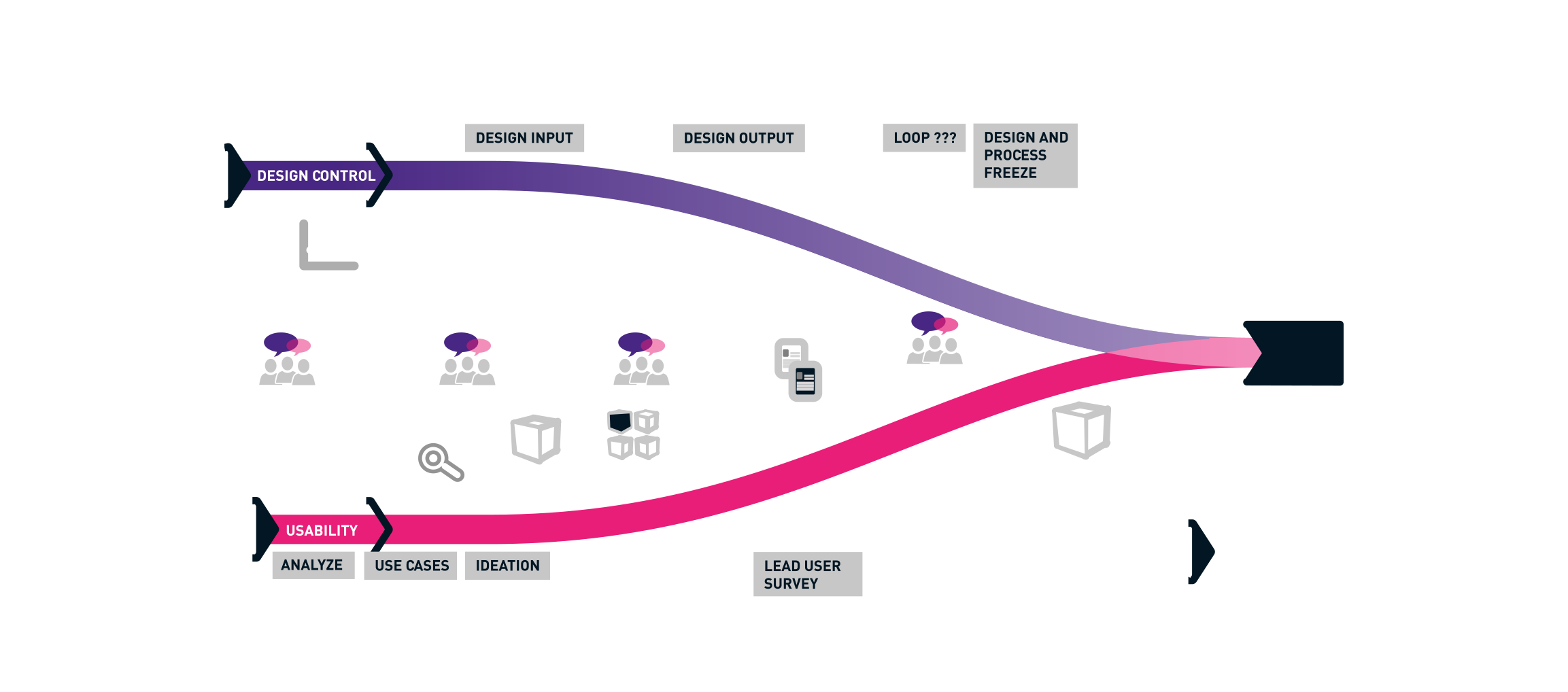
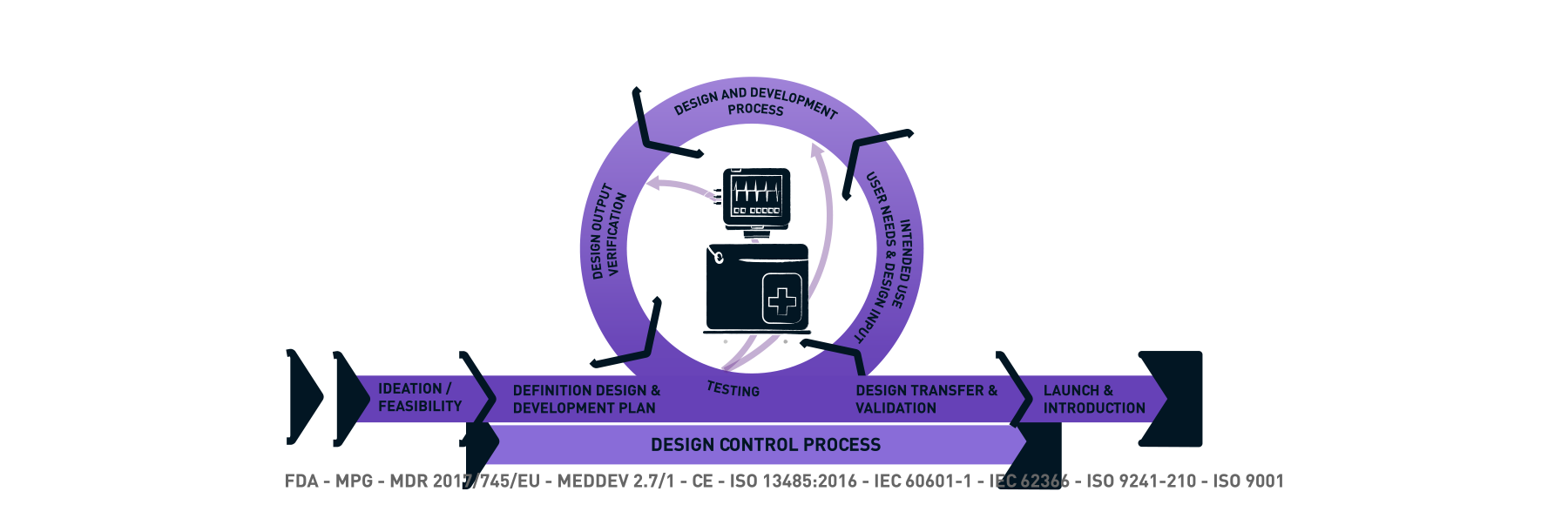
MEDICAL 720° - The BUSSE method for successful medical products
We look at the development of your products from both a hardware and a software perspective. That is why we have taken the Human Centered Design Process a step further and literally go 2x around a product during development: with the user-oriented view of a UI / UX designer and from the three-dimensional-functional view of the industrial designer and engineer. In this way, an optimal operating and ergonomic concept go hand in hand and not only well thought-out and operable products are created, but also products that leave a positive, brand-defining experience through professional design. Usability and optimum operability and process reliability are therefore a central component. A normative requirement for medical devices.
Success with formative and summative usability tests
Case Study Medical Usability 720°